Office of Regulatory Research Compliance
Howard University
HU Research Bldg 1
1840 7th Street, NW
Suite 309
Washington, D.C. 20001
Phone: (202) 865-8597
Fax: (202) 232-5286
ORRC is located directly across from the Howard University Shaw Metro on 7th Seventh St.(Georgia Ave) on the third floor of the Howard University Research Building-1.
TYPES OF SUBMISSION
KEY DEFINITIONS
- Minimal Risk: “Minimal risk means that the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.” See the Common Rule at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.102.
- Further Guidance on what categories of research would meet minimal risk definition can be found at http://www.hhs.gov/ohrp/policy/expedited98.html.
GREATER THAN MINIMAL RISK STUDIES
Studies that do not qualify as "minimal risk" nor for expedited review under the federal regulations are usually considered greater than minimal risk. Greater than minimal risk studies usually involve medical procedures or devices or create some high degree of discomfort for participants. This discomfort can be physical, emotional, social, or psychological. Studies in this category should be completed on the following forms:
- A1 Form: For Greater Than Minimal Risk Studies
- A2 Form: Continuation/Renewal Form For Greater Than Minimal Risk Studies
Please see the checklists in the IRB Forms and Tools section to determine all the necessary documents that should be submitted with your application
CHART REVIEWS, CANCER REGISTRY, AND MEDICAL DATA REQUESTS
Requests to review medical information or charts fall within this category. The major of studies will require HIPAA authorization to review protected health and medical information. PI's working with this type of information should also complete the compliance training modules conduct by the Compliance Office in the Howard University Hospital. Studies in this category should complete one of the following forms:
- B1 Form: For Greater Than Minimal Risk Studies
- B2 Form: Continuation/Renewal Form For Greater Than Minimal Risk Studies
- Cancer Registry Forms
All researchers who wish to access data from the Rosemary Williams Cancer Registry (HU Cancer Center Registry) must first complete the Pre-Screening Interview Form in conjunction with the Manager of the Register, Ms. Alfreda Woods. The approved data request form is submitted to the IRB along with the appropriate level data application. For more information on the cancer center registry, please click the link to the Rosemary Williams Cancer Registry.
MINIMAL RISK STUDIES
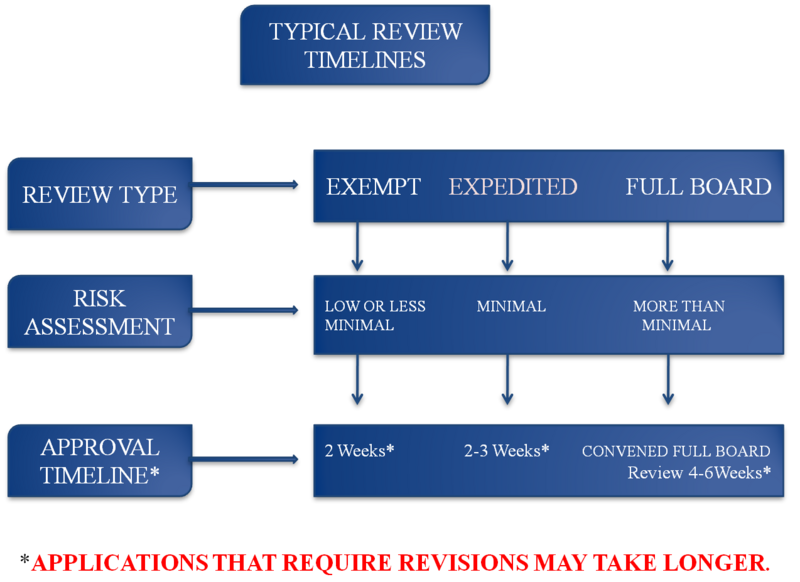
Most of the psychosocio-behavioral qualifies as a minimal risk study, as defined above according to 45 CFR 46. Studies that pose minimal risk to human participants can often be processed through the "expedite review" pathway. A designated member of the Board can review submissions for approval in those cases. The expedited review process takes from two to three weeks (2-3 weeks). This process should not be viewed as "hurried" review. Expedited reviews get the same attention as any protocol submitted to the full Board.
Minimal risk studies that involve vulnerable populations (children, pregnant women, fetuses and neonates, human in vitro fertilization, prisoners, economically or educationally disadvantaged participants, cognitively impaired persons) must go before the full Board for consideration.
Most student research for theses/dissertations will fall under this category.
EXEMPT STUDIES AND SECONDARY DATA ANALYSIS
There are six criteria for exemption. Only the IRB can make the official determination that your project is exempt based upon 45 CRF 46. 101:
(1) Research conducted in established or commonly accepted educational settings involving normal educational practices, such (i) research on regular and special education instructional strategies, or (ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods
(2) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior unless: (i) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to subjects; and (ii) any disclosure of the responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation.
(3) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement) survey procedures, observation of public behavior that is not exempt under paragraph b2 of this section, if: (i) the human subjects are elected or appointed public officials or candidates for public office; or (ii) federal statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter.
(4) Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
(5) Research and demonstration projects which are conducted by or subject to the approval of department or agency heads, and which are designed to study, evaluate, or otherwise examine: (i) public benefit or service programs; (ii) procedures for obtaining benefits or services under those programs; (iii) possible changes in or alternatives to those programs or procedures; or (iv) possible changes in methods or levels of payment for benefits or services under those programs.
(6) Taste and food quality evaluation and consumer acceptance studies, (i) if wholesome foods without additives are consumed or (ii) if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved y the Environmental Protection Agency or the Food and Safety and Inspection Service of the U.S. Department of Agriculture.
Institutional Review Board (IRB)
Office of Regulatory Research Compliance